What The Research Shows
LACRIFILL® Canalicular Gel has been cleared for use by the FDA based on the results of clinical studies showing it’s safe for patients and effective at keeping natural tears in the eye. See ResultsProof-of-Concept Study
A pilot study of LACRIFILL Canalicular Gel involving 63 patients with dry eye found there was an increase in tears remaining on the eye and an increase in tear volume, lasting for at least 3 months.
%
of patients had no pain
%
of patients had no infections
%
reported their eyes felt better
Fezza JP, Cross-linked hyaluronic acid occlusive device for the treatment of dry eye syndrome, Clinical Ophthalmology 2018_12: 2777-2283
Clinical Trial Results
The findings below were published in the October 2024 issue of the Journal of Cataract and Refractive Surgery (JCRS) in the “Effectiveness and safety of a novel crosslinked hyaluronate canalicular gel occlusive device for dry eye” article.
Based on the Pivotal Study
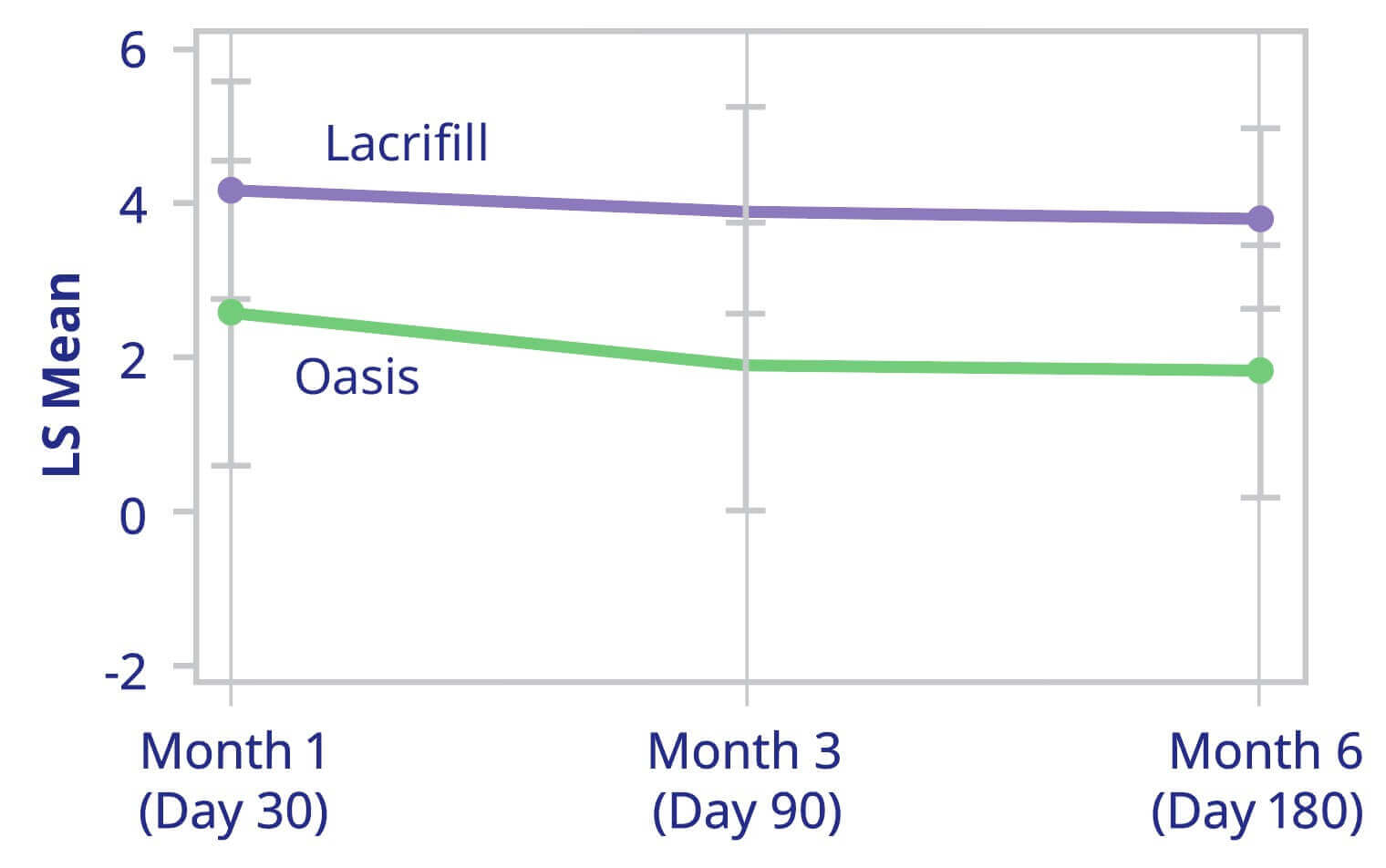
Change from Baseline of Anesthetized Schirmer’s Test (Averages of Both Eyes) at all Post-Baseline Visits
ITT Population with Available Data Only
ITT Population with Available Data Only
Average of Both Eyes Anesthetized Schirmer’s Test
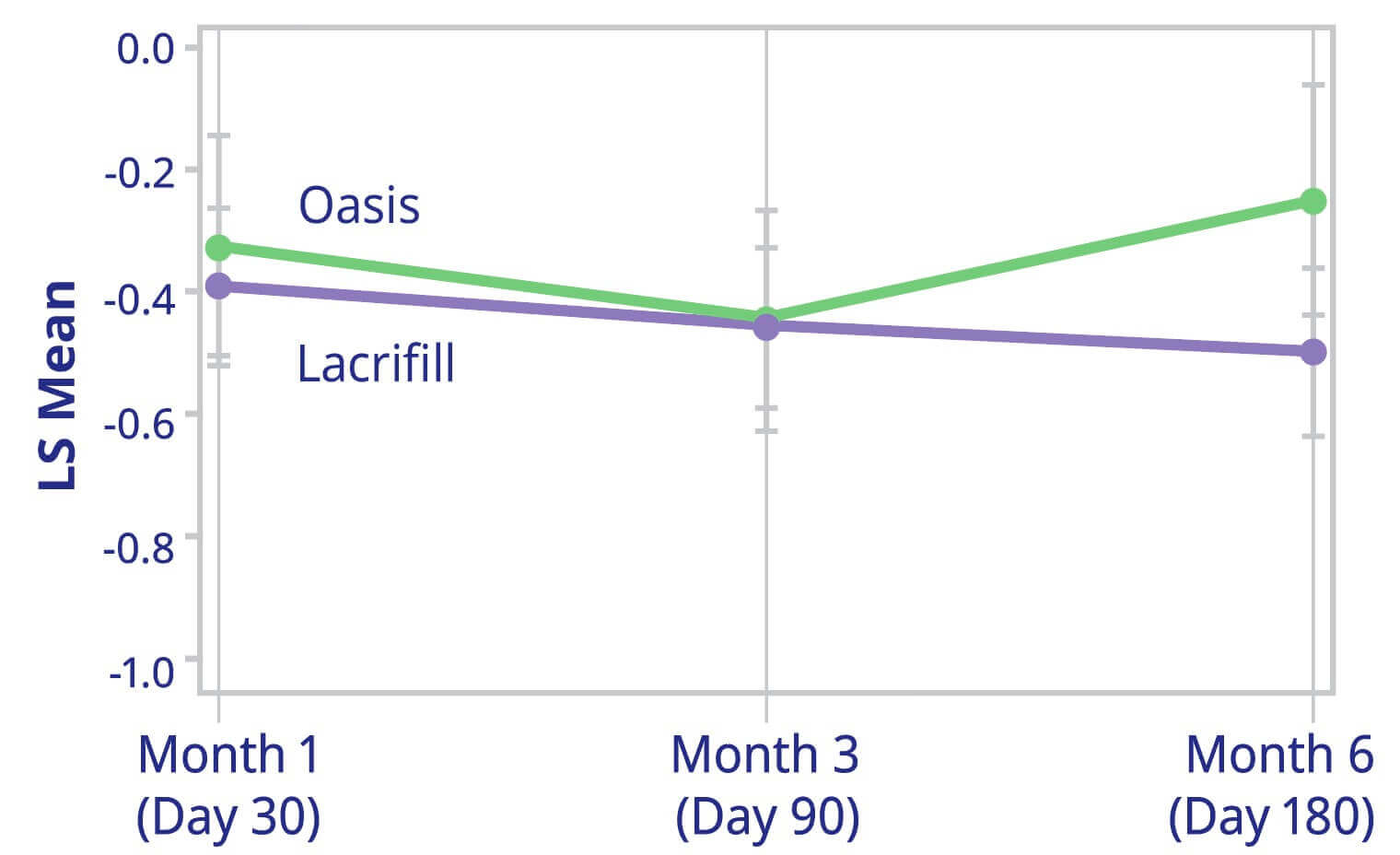
Change from Baseline of Corneal Fluorescein Staining
(NEI Scale; Averages of Both Eyes) at all Post-Baseline Visits
ITT Population with Available Data Only
(NEI Scale; Averages of Both Eyes) at all Post-Baseline Visits
ITT Population with Available Data Only
Average of Both Eyes Fluorescein Staining NEI Scale – Central
Planned Treatment:
Oasis Form Fit Hydrogel Canalicular Plug
Lacrifill Canalicular Plug
Packer M, Lindstrom R, Thompson V, Parekh JG, Gupta P, Nijm LM, Donnenfeld E. Effectiveness and safety of a novel crosslinked hyaluronate canalicular gel occlusive device for dry eye. J Cataract Refract Surg. 2024 Oct 1;50(10):1051-1057.
doi: 10.1097/j.jcrs.0000000000001505. PMID: 38875184
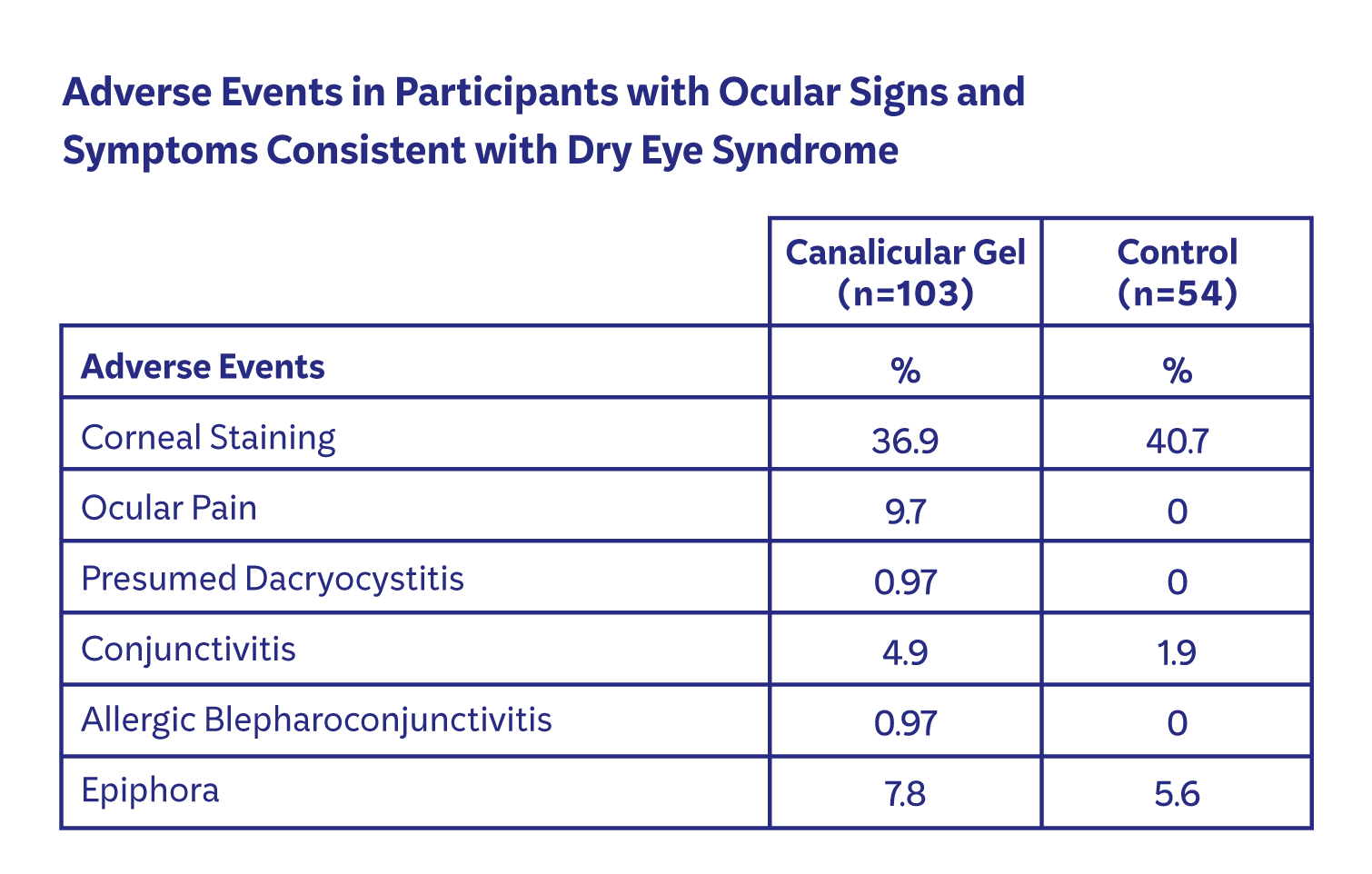
For a full listing of Adverse Events associated with LACRIFILL Canalicular Gel, please see lacrifill.com/instructions-for-use.
